The Project Outcomes Report for our recently ended NSF grant (known variously as DEB-1723293, DEB-1457701, and DEB-1456652) is now available on grants.gov:
Life comes in two forms: single-celled (organisms made up of only one cell) and multi-celled (organisms made up of many cells, alike or different). Multicellular life has evolved from unicellular ancestors many times across the tree of life, and the resulting radiations have transformed nearly every ecosystem on Earth. Ancestors of animals, plants, fungi, several groups of seaweeds, and filamentous bacteria underwent the transition from single- to multi-celled life in the deep past. While each of these origins is a replicate experiment with the potential to inform our understanding of how and why multicellular life evolved, the window through which we see these ancient events is blurry. Extinctions, subsequent evolution, and a spotty fossil record obscure our view. Experimental evolution enables us to time-travel, making it possible to clearly observe the evolution of multicellularity as it occurs in the lab. This project integrated experimental, bioinformatic, theoretical, and comparative approaches to understand how multicellularity and related traits have evolved, and how they can evolve.
The evolution of multicellularity is an example of an evolutionary transition in individuality. In unicellular species, the individuals that make up a population are cells; in multicellular species, the individuals that make up a population are groups of cells. When a unicellular population evolves to become multicellular, the unit we consider to be an individual changes from a single cell to a multicellular organism. To model how this shift in individuality occurs as multicellularity evolves, we used the volvocine green algae (multicellular Volvox, unicellular Chlamydomonas, and their relatives).
We previously showed that Chlamydomonas reinhardtii could evolve different multicellular structures under selection for an increased rate of settling in liquid medium. In this project, we used a combination of genomics, quantitative genetics, and transcriptomics to explore the genetic basis of this initial step towards multicellularity. We discovered that compared to unicellular forms, multicellular algae exhibited widespread changes in the expression of many genes, especially those related to the cell cycle and to reproductive processes. Overrepresented among these significantly altered genes were those that were C. reinhardtii-specific and volvocine-specific. This last observation suggests that the genetic basis for multicellularity evolved in the lab has more in common with Chlamydomonas‘ relatives among the volvocine algae than with other multicellular green algae or land plants.
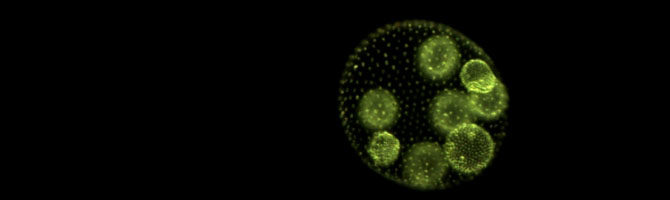
We then performed a different kind of selection experiment to test whether predation could also drive unicellular organisms to evolve a multicellular life cycle. We cultured replicate populations of C. reinhardtii either in the presence or in the absence of a filter-feeding, single-celled predator, Paramecium tetraurelia. In two of the populations exposed to predation, the algae evolved multicellular spheroids of 4-16 cells. Detailed observations of the evolved life cycles showed that some isolates pass through a unicellular stage, while some remain multicellular for their entire life cycle. Paramecium preys on unicellular Chlamydomonas efficiently, but the larger multicellular algae are much less likely to be eaten. This protection against predation give the multicellular algae a fitness advantage, and it is likely that this is what drove them to evolve multicellularity.
Using analytical and simulation models, we showed that evolutionary transitions to a new level of individuality, such as from unicellular to multicellular organisms, result in an increase in trait heritability under a wide range of conditions. For a trait to evolve under natural selection, it is essential that the trait have heritable variation within the population. Nascent multicellular organisms necessarily have traits that were not present in their unicellular ancestors (the size of the multicellular group, for example), and the heritability of these novel traits has previously been presumed to be low, presenting a problem for their continuing evolution. For clonal populations, we showed that this is not the case; in fact, the heritability of multicellular-level traits is often higher than that of the unicellular-level traits from which they arise.
To date, the project has produced nineteen peer-reviewed publications, with further papers in development. Newly-generated whole genome and whole transcriptome sequences have been made publicly available. Several graduate and undergraduate students, high school students, and a postdoc were trained in experimental evolution, bioinformatics, and evolutionary theory. In addition, two high school teachers and a graduate student were involved in developing and implementing an experimental evolution curriculum, and the teachers presented this work at the American Science Teachers Association meeting in 2017.
Herron, M.D., Borin, J.M., Boswell, J.C., Walker, J., Chen, I.-C.K., Knox, C.A., Boyd, M., Rosenzweig, F., & Ratcliff, W.C. 2019. De novo origins of multicellularity in response to predation. Sci. Rep., 9: 2328. doi: 10.1038/s41598-019-39558-8